Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Assessment of Possible Link Between Uropathogenic Escherichia Coli and Azoospermia in Male Mice
*Corresponding author:Vijay Prabha, Department of Microbiology, Panjab University, Chandigarh-160014, India
Received:February 03, 2023; Published:February 23, 2023
DOI: 10.34297/AJBSR.2023.18.002433
Abstract
Controversies and debates still prevail in current literature concerning the role of bacterial infection in male infertility. A handful of studies have discovered a correlation between bacteria and azoospermia condition in which the male discharge is utterly devoid of spermatozoa. In line with this, the present study was designed to evaluate the influence of Escherichia coli on spermatogenesis. A single dose (108 cfu/20ul) of uropathogenic Escherichia coli causing sperm agglutination was administered intratesticularly and animals were euthanized on Day 7. Various parameters viz. body weight profile, TSI (%), bacterial load, spermiogram analysis, histopathological changes were evaluated in comparison to the control group administered with PBS. The administration of E. coli led to impairment of spermatogenesis as evident by absence of spermatozoa in right side of testis and revealed by the histopathological examination of the testes showing altered histology. The histology depicted hypospermatogenesis with significant alterations in tissue architecture with no spermatozoa as compared to the control group, indicating azoospermia. If E. coli is injected into the testicles, it can colonise there and eventually lead to azoospermia in the male. This research provides new perspectives and allude to a potential explanation by which uropathogen infection reduces a man’s reproductive vitality and causes long-term damage to the male reproductive system.
Keywords: Escherichia coli, Infertility, Sperm agglutinating
Introduction
The vitality of a species depends on its ability to reproduce successfully. On the other hand, statistics of dwindling male fertility have been on the ascent lately. About 15% of male infertility cases are caused by infections in the reproductive system. Because they alter semen parameters negatively and may evolve to azoospermia, in which the male discharge is utterly devoid of spermatozoa, infections of the male genital tract are one of the major aetiological concerns in male reproductive health. About 15% to 20% of infertile men have azoospermia, which significantly reduces their chances of fatherhood [1]. Obstructive azoospermia (OA) and non-obstructive azoospermia (NOA) are 2 potential classifications for this disorder. Disruption of normal testicular function and the seminal tract is what the former term alludes to, whereas spermatogenesis failure is what the later term describes [2]. The most severe form of male infertility is known as non-obstructive azoospermia (NOA), and it is characterized by quantifiable damage to spermatogenesis, which includes three testicular pathophysiological phenotypes (Sertoli cell-only syndrome [SCOS], maturation arrest [MA], and hypo spermatogenesis) [3, 4].
Infections in the male reproductive system have been linked to both Gram-positive and Gram-negative bacteria. Gram-negative bacterium uropathogenic Escherichia coli has been identified as a key player in male infertility; research findings have indicated that this bacterium occurs as the most frequently detected microbe from the seminal fluid of infertile men, and that it is responsible for between 60 and 85% of cases of persistent bacterial prostatitis that ultimately impair spermatozoa [5]. Multiple virulence factors (VFs) are available in Escherichia coli and are used for host attachment, invasion, and damage. Through the production of cytokines and reactive oxygen species, immediate interaction or mediation by its soluble metabolites reduces sperm motility and mitochondrial membrane potential. Only a limited fraction of in-vitro research has confirmed the significant role played by E. coli [6].
As shown by the findings, microbial infections are linked to the emergence of azoospermia. There have been several reports on the impact of microbial infections on OA, but little research has focused on the bacteria linked to NOA. However, there are major unexplored avenues in the connection between bacteria and NOA. While much research has focused on the role of genetic variables in NOA-such as chromosomal abnormalities-surprisingly little has investigated the possible link between microbes and NOA [7]. So, the goal of this study is to investigate the link between Escherichia coli and NOA to see if there is any possible link to the condition of azoospermia.
Material and Methods
Animals
Sexually mature, male BALB/c mice, 5-6 weeks old, 25±2 g, were procured form the Central animal house of Department of Microbiology, Panjab University, Chandigarh, India. The animals were given standard pellet food (M/s Ashirwad Industries Pvt. Ltd.) and water ad libitum. The prospective study procedures were approved by Institutional Animal Ethics Committee, Panjab University; Registration No. IBSC-PANUNI-077-2020 and the experiments were carried out ensuing the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Microorganism
The clinical strain of Escherichia coli used in the present study was isolated earlier in our laboratory from semen sample of infertile male, undergoing analysis at special infertility clinic at Department of Urology, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. The strain causing 100% sperm agglutination in vitro was maintained on Luria agar and was stored as glycerol stock at -80°C.
In vivo Effect of Single Dose of Escherichia coli on Mouse Reproductive Parameters
Inoculum preparation and experimental groups:Escherichia coli was cultivated in 10ml Luria broth (LB) at 37°C/ 24h.The cell culture was centrifuged at 10,000g for 20min. The pellet obtained was washed twice with Phosphate Buffer Saline (PBS) (50mM, pH 7.2) and the bacterial cells were resuspended in the same buffer. The serial dilution method was performed to attain a cell count of 108 cfu/20μl. Male BALB/c mice were divided into two groups; Group I was inoculated with 20μl of PBS and Group II was administered with 108 cfu/20μl PBS/mouse of sperm agglutinating Escherichia coli.
Intratesticular administration:The intratesticular inoculation was carried out under surgical conditions wherein mice were anesthetized (ketamine and xylazine). Under sterile conditions, the mice were placed in supine position and disinfected with 80% ethanol. The abdominal area was gently pressed to protrude out the testis and inoculated with a single dose of 20μl (Escherichia coli/PBS).
Body weight profile and Tissue Somatic Indices TSI (%):The initial and final body weight of mice from each group was taken on all the respective days of authorization. Mice were sacrificed by cervical dislocation on Day 7 and testes, caudal epididymis, vas deferens of right side were collected, weighed and TSI (%) was calculated.
Bacterial load of tissues:After sacrifice, one half of the collected organs dissected under sterile conditions were weighed and homogenized manually in PBS buffer. The bacterial load was calculated in terms of log cfu/g of tissue.
Analysis of spermiogram: Under sterile conditions, the vasa deferentia of mouse were gently pulled out and each vas deferens was placed in a glass plate containing pre-warmed 250μl of freshly prepared PBS buffer. The vas deferens was gently squeezed to extract the spermatozoa into the buffer. The sperm samples were collected and kept at 37°C, following which the spermiogram analysis in terms of sperm count, motility (%), viability (%) and morphology were evaluated.
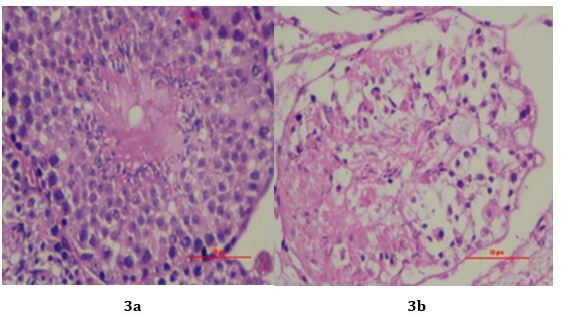
Histopathological analysis:Histopathological analysis of testis from the right side was carried out by hematoxylin-eosin staining. By using standard histological procedure, organs of mice were grossly removed and fixed in 10% buffered formaldehyde for 24 h. After 24 h, the tissues were further treated with 70- 90% alcohol for dehydration and then embedded in paraffin. The sections of 4 mm paraffin tissue were stained with hematoxylineosin stains (H & E). The slides were envisaged at 100X and 400 X magnifications for any observable alterations in the tissues.
Results
In vivo Effect of Single Dose of Escherichia coli on Mouse Reproductive Parameters
Body weight and Tissue Somatic Indices (%):Group I administered with PBS revealed a 5.36% increase in body weight and Group II which received 108 cfu of E. coli depicted 3.95% decrease in the body weight of mice, on Day 7 (Figure 1).
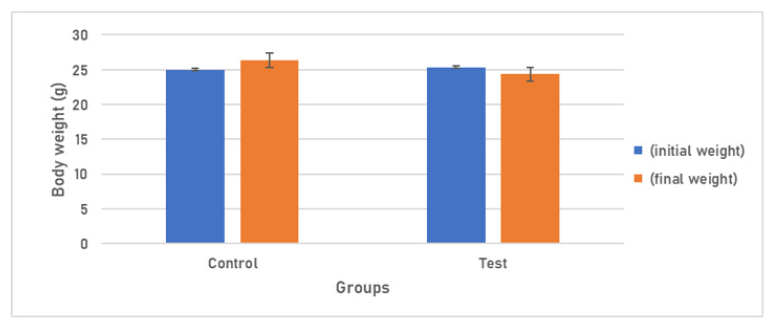
Figure 1: Body weight response of Group I am receiving 20μl of PBS and Group IV receiving 108 cfu/20μl of E. coli.
In the case of Group I, TSI (%) of testis (0.27±0.08), epididymis (0.06±0.02), vas deferens (0.024±0.002) were recorded. However, in Group II, significant changes in TSI (%) levels for the testis (0.5±0.15), epididymis (0.25±0.137), vas deferens (0.04±0.024) were detected (Figure 2).
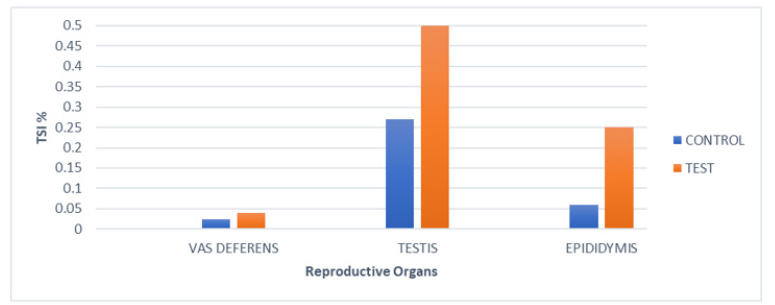
Figure 2:Tissue somatic indices (%) of various reproductive organs of mice of Group I (PBS) and of Group II after Intratesticular inoculation, on day7.
Bacterial load of tissues:The homogenates of reproductive organs showed that all the organs were bacteriologically sterile in the case of group I. In contrast, reproductive organs of right-side of group II mice were efficiently colonized with E. coli (Table 1).
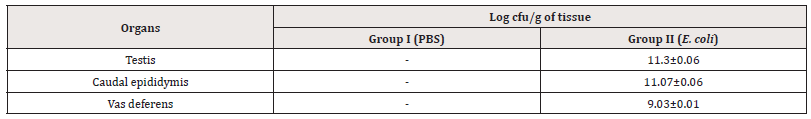
Table 1:Enumeration of bacterial load (log cfu/g) from testis, cauda epididymis and vas deferens of mice on day 7 after intratesticular administration of E. coli.
Note*: (-) No viable count; [values represent Mean±SD].
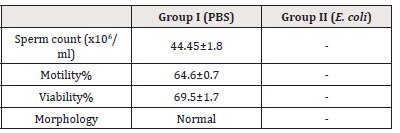
Note*: (-) No sperm count; [values represent Mean ± SD]
Analysis of spermiogram: Sperm count, Motility%, Viability%,
Morphology of spermatozoa from both Group I and Group II were
noted (Table 2).
i. Sperm count, Motility%, Viability%: The sperm count in
Group I, administered with PBS, showed 44.45x106/ml count,
percentage motility of spermatozoa to be 60.57% and viability
was found to be 69.53% While, it is worth mentioning that the
test group exhibited absence of spermatozoa.
ii. Morphology: The morphology of spermatozoa was normal
for Group I. In Group II, no spermatozoa were observed.
Histopathological analysis:The right testis administered with single dose of E. coli obtained from mice of Group I and II, were analysed for histopathological alterations on day 7. The histopathological examination of testis of control group revealed no clinical sign of histological alterations as regular spermatogenesis was observed. Testis was normal, showing clusters of spermatozoa in the Centre surrounded by layers of germinal cells. Mature spermatids and mature spermatozoa were observed (Figure 3a). Compared to the control group, Group II had histologically altered tissues. Degeneration and necrosis were seen in the germ cell layers. The absence of mature spermatozoa and spermatids in the testis was indicative of azoospermia (Figure 3b).
Discussion
The complete absence of spermatozoa in the ejaculate makes azoospermia a substantial male reproductive condition. The two most frequent types of azoospermia, obstructive azoospermia (OA) and non-obstructive azoospermia (NOA), have their own unique etiologies and manifestations [8]. Some investigations have shown that uropathogens are critical in the development of both OA and NOA.
Escherichia coli, Staphylococcus aureus, Streptococci, Enterococci, Pseudomonas aeruginosa, Serratia marcescens, Proteusmirabilisand Candida albicans are the most frequently reported uropathogens. Male reproductive health may be compromised by urinary tract infections. Infections of the urogenital system may hinder male reproduction in two ways: first, by causing an injury that triggers an inflammatory response; and second, by interfering with the normal functioning of the male reproductive organs [8]. Mice were administered intratesticularly with 108 cfu/20μl of bacteria to ascertain the underlying aetiology of NOA in males by elucidating the influence of uropathogenic microorganisms on lower reproductive potential. On day 7, the mice were euthanized, and their weight profile, TSI (percent), sperm parameters, and histological changes were analyzed.Throughout this study, mice in the Control group gained weight, but mice injected with a single dose of E. coli lost weight. As weight variance is reliant on the equilibrium between energy utilization and nutritional consumption, the mice in the Test group’s losing weight may be ascribed to a decrease in food intake triggered by sickness. These results are similar to van Heeckeren, et al’s. (2000) study, which underlined the impact of Pseudomonas infection and revealed a considerable losing weight three days after infection [9]. Similar results were obtained by Wan, et al. (2016), who discovered that mice administered an intravenous dosage of E. coli progressively decreased body weight [10].
The TSI% of reproductive organs (testes, cauda, and vas deferens) was also measured to evaluate their degree of functionality under various experimental scenarios. The anatomical and physiological abnormalities of the right set of organs, which may be attributed to infection of the urogenital tract, resulted from abrupt changes in the TSI levels of the right set of organs. Furthermore, PBS-infected mice exhibited no notable alterations in the TSI values of the various reproductive organs. These results are in line with those reported by Jantos, et al. (1992), who observed that administering C. trachomatis biovar mouse pneumonitis through intravenous injection induced significant alterations in the relative organ weights and an enlargement of the epididymites [11]. The bacterial load of uropathogenic microorganisms from the reproductive tissues (Testis, cauda, and vas deferens) of mice was determined to trace the bacteria. As expected, mice administered with PBS demonstrated no viable count.
The basic variables, including sperm count, motility, viability, and morphology, were also evaluated to ascertain the impact of uropathogen invasion on the spermiogram of mice. Additionally, the present study found that all groups given sperm agglutinating E. coli had an adverse effect on seminal parameters versus the control group given PBS. Correlations between decreased sperm concentration, motility, and viability suggest infection as the only cause of the response. This is supported by findings from the work of Jantos, et al. (1992), who observed inflammation in the testes of rats and documented a moderate to extensive loss of sperm production 14 days after inoculation with Chlamydia psittacine [11]. In addition, Sellami, et al. (2014) revealed that male Swiss mice infected with 106 cfu with C. trachomatis in the meatus urethra had decreased reproductive effectiveness as determined by modifications to seminal parameter values [12]. Exposure of human spermatozoa to 106 cfu/ml of viable Escherichia coli, as shown in a 2017 by Cano, et al., significantly reduces sperm motility [13]. In the present study, mice in the test groups showed severe abnormalities in their reproductive organs on histopathological examination, whereas mice in the control group/PBS showed no alterations in the histological structure of organs. Histological analysis revealed that the seminiferous epithelium of the right testis had stopped producing sperm. This severe spermatogenesis failure may have been caused by the loss of germ cells and harm to somatic cells in the testicles. These findings are consistent with those from the research by Hanna, et al., 1993, which showed that the use of calcium antagonists had a detrimental effect on spermatogenesis in rats [14].
Conclusion
Based on the findings of the current study, it can be concluded that administration of E. coli via intratesticular route led to their colonization in the male reproductive tract. As a result, spermatogenesis and sperm parameters were shown to be negatively affected by these bacteria. A further demonstration of azoospermia in the testes of male mice was provided by histological investigation, corroborating the aforementioned results. All of these findings of our study, provides novel insights and indicates that the lower reproductive vigour in men infected with uropathogens may potentially be mediated, eventually causing harm to the male reproductive system. However, further studies need to be carried out to obtain molecular mechanisms behind this observed effect and gather conclusive evidence in support of this hypothesis.
Conflicts of interest
None.
References
- Caroline Kang, Nahid Punjani, Peter N Schlegel (2021) Reproductive Chances of Men with Azoospermia Due to Spermatogenic Dysfunction. J Clin Med 10(7): 1400.
- W Weidner, W Krause, M Ludwig (1999) Relevance of male accessory gland infection for subsequent fertility with special focus on prostatitis. Hum Reprod Update 5(5): 421-432.
- Elizabeth Hervey Stephen, Anjani Chandra (2006) Declining estimates of infertility in the United States: 1982-2002. Fertil Steril 86(3): 516-523.
- Laura Kasak, Maris Laan (2021) Monogenic causes of non-obstructive azoospermia: challenges, established knowledge, limitations and perspectives. Hum Genet 140(1): 135-154.
- Veronica Folliero, Marianna Santonastaso, Federica Dell'Annunziata, Pasquale De Franciscis, Giovanni Boccia, et al. (2022) Impact of Escherichia coli outer membrane vesicles on sperm function. Pathogens 11(7): 782.
- Parvin Sabeti, Soheila Pourmasumi, Tahereh Rahiminia, Fatemeh Akyash, Ali Reza Talebi (2016) Etiologies of sperm oxidative stress. Int J Repro Biomed 14(4): 231-240.
- Tao Luo, Qian Xing Zou, Yuan Qiao He, Hua Feng Wang, Tao Wang, et al. (2016) Matrine compromises mouse sperm functions by a [Ca(2+)]i-related mechanism. Reprod Toxicol 60: 69-75.
- Matthew Wosnitzer, Marc Goldstein, Matthew P Hardy (2014) Review of Azoospermia. Spermatogenesis 4: e28218.
- AM van Heeckeren, JTscheikuna, RW Walenga, MW Konstan, PB Davis, et al. (2000) Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am J Respir Crit Care Med, 161(1): 271-279.
- Taomei Wan, Guiqiang Yuan, Yi Ren, Zhicai Zuo, Zhengyi Wang, et al. (2016) Diet-induced obese mice exhibit altered immune responses to acute lung injury induced by Escherichia coli. Obesity (Silver Spring) 24(10): 2101-2110.
- C Jantos, W Baumgärtner, B Durchfeld, HG Schiefer (1992) Experimental epididymitis due to Chlamydia trachomatis in rats. Infect Immun 60(6): 2324-2328.
- Hanen Sellami, Abir Znazen, Afifa Sellami, Hela Mnif, Nour Louati, et al. (2014) Molecular detection of Chlamydia trachomatis and other sexually transmitted bacteria in semen of male partners of infertile couples in Tunisia: the effect on semen parameters and spermatozoa apoptosis markers. PloS one 9(7): e98903.
- Cano Cháves A, Galarzo Pardo S, Puerta Suárez J, Giraldo M, Cadavid à P, et al. (2017) Effect of uropathogenic bacteria and soluble factors of their metabolism on sperm quality: Escherichia coli and Enterococcus faecalis. Clinic and Research in Gynecology and Obstetrics 44: 106-112.
- PM Hanna, MB Kadiiska, SJ Jordan, RP Mason (1993) Role of metallothionein in zinc (II) and chromium (III) mediated tolerance to carbon tetrachloride hepatotoxicity: evidence against a trichloromethyl radical-scavenging mechanism. Chem Res Toxicol 6(5): 711-717.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.